
Summary
SpectrometrySpectrometry is the observation and measurement of wavelengths of light or other electromagnetic radiation. Once a range of wavelengths, or a spectrum, for a gas mixture has been measured, by… Learn more… with GC (Gas Chromatograph) and MS (Mass Spectrometry) is a laboratory based analytical technique that combines the features of gas-chromatography and mass spectrometry to identify different substances within a gas sample extracted from a flare line to determine its composition.
How it Works
The GC-MS method is composed of two major components, these are the gas chromatograph and the mass spectrometer.
The gas chromatograph utilises a capillary column which separates the molecules as the sample travels the length of the column. The molecules are retained by the column and then elute (come off) from the column at different times (called the retention time), this then allows the mass spectrometer downstream to capture, ionise, accelerate, deflect, and detect the ionized molecules separately.
The mass spectrometer does this by breaking each molecule into ionised fragments and detecting these fragments using their mass-to-charge ratio.
These two components, used together, allow a much finer degree of molecule identification than either unit used separately.
It is not possible to make an accurate identification of a particular molecule by gas chromatography or mass spectrometry alone.
The mass spectrometry process normally requires a very pure sample while gas chromatography using a traditional detector (e.g., flame ionisation detector) cannot differentiate between multiple molecules that happen to take the same amount of time to travel through the column (i.e., have the same retention time), which results in two or more molecules that co-elute.
Sometimes two different molecules can also have a similar pattern of ionised fragments in a mass spectrometer. Combining the two processes reduces the possibility of error, as it is extremely unlikely that two different molecules will behave in the same way in both a gas chromatograph and a mass spectrometer.
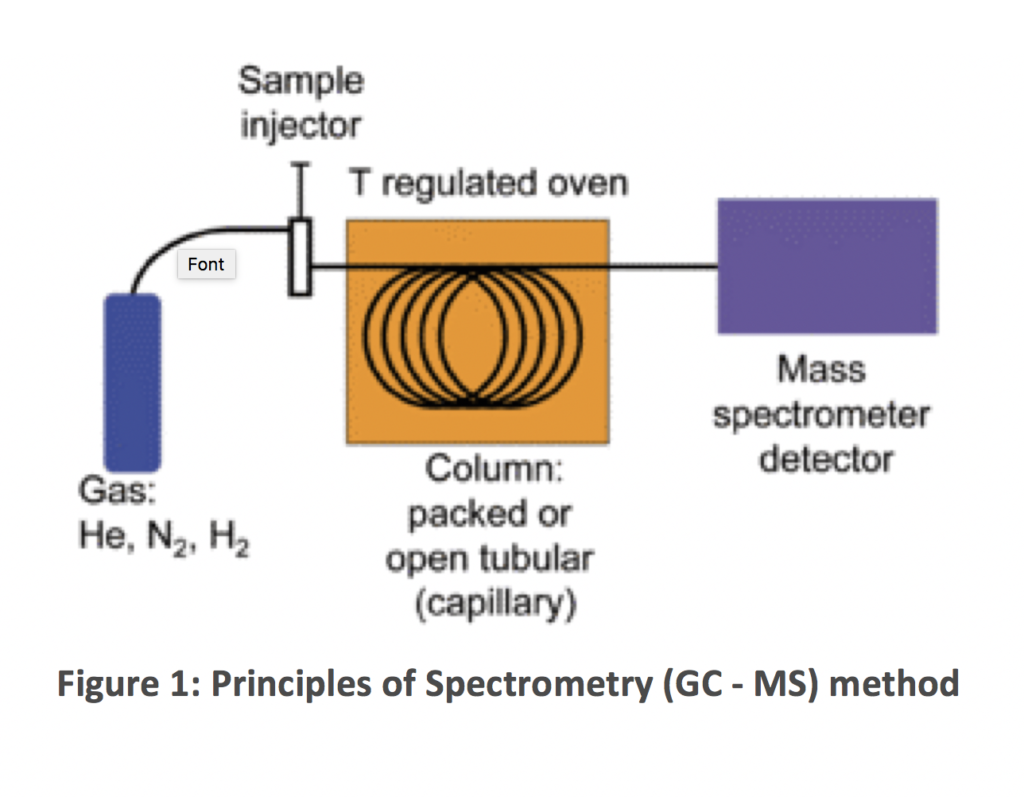
Spectrometry (GC – MS) is not widely used in the Oil and Gas Industry.
Advantages
High accuracy
Low uncertainty
Limitations
Mainly laboratory based method
Periodic update (daily, weekly, monthly)
High equipment costs
Go Deeper
Case study
No case study available at this time.
On-line analytical methods involve extracting a sample from a flare system via an automatic sampling system and analysing it continuously with a field installed analyser system.